Price fixation/revision of prices of scheduled formulations as listed in Schedule I of the Drug (Prices Control) Order is the key task entrusted to the National Pharmaceutical Pricing Authority (NPPA). In addition to this, two main functions of the NPPA are: Monitoring and enforcement of the notified prices and providing inputs to the Government for policy formulation and other specific issues related to availability, accessibility and affordability of medicines for all. On July 16, 2020 guidelines were issued by the NPPA for setting up Price monitoring and Resource Units (PMRUs) at the State/Union Territories under the Central Sector Scheme of Consumer Awareness, Publicity and Price Monitoring. It was felt that jobs such as collection, compilation/creation and analysis of market-based data must become an integral part of the working of the NPPA. It was desirable under the National Pharmaceutical Pricing Policy 2012 and the Drug (Prices Control) Order 2013 too. To achieve this, the support from the States/Union Territories is necessary through the setting up of Price Monitoring and Resource Units (PMRUs).
PMRUs were expected to function under the direct supervision of the concerned State Drug Controller and were expected to be key collaborating partners of the NPPA with information gathering mechanism at the grassroot level. The objectives behind setting up of the PMRUs included monitoring of notified prices of medicines, detection of violation of the provisions of the DPCO, pricing compliance, ensuring availability of medicines, monitoring of the price movement of scheduled and non-scheduled formulations, revision of price of scheduled formulations by the manufacturer based on the annual increase in the Wholesale Price Index (WPI), overseeing the price non-scheduled formulations as prices of such formulations are not allowed to increase beyond 10 per cent on annual basis. Additionally, PMRUs were entrusted the task of collection and compilation of market-based data of scheduled as well as non-scheduled formulations and analyse them, collection of test samples of medicines at the retail market whenever required, conducting training, seminars and workshops at the State and District levels for consumer awareness and publicity covering aspects relating to the role and functions of the NPPA, among others.
Mandate for PMRUs is very comprehensive and to perform the role effectively, PMRUs have to constantly interact with different stakeholders comprising State government department, State Drug Controllers, consumer groups, and public at large. State/Union Territories (UTs) have been divided into three categories namely Category I, II and III. Category I comprised States/UTs having population of more than 3 per cent of the total population and included Andhra Pradesh, Bihar, Gujarat, Karnataka, Madhya Pradesh, Maharashtra, Odisha, Rajasthan, Tamil Nadu, Uttar Pradesh, and West Bengal. Category II comprised States/UTs having population of less than 3 per cent but more than 1 per cent of total population and comprised Assam, Chhattisgarh, Delhi, Haryana, Jammu & Kashmir, Jharkhand, Kerala, Punjab, and Telangana. Category III comprised States/UTs having population of less than 1 per cent of the total population and included Andaman & Nicobar Islands, Arunachal Pradesh, Chandigarh, Dadra & Nagar Haveli and Daman & Diu, Goa, Himachal Pradesh, Ladakh, Lakshadweep, Manipur, Meghalaya, Mizoram, Nagaland, Puducherry, Sikkim, Tripura, and Uttarakhand.
Table 1: Year-wise PMRUs registered
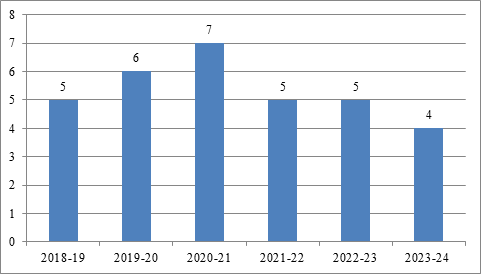
Source: Compiled from Integrated Pharmaceutical Database Management System 2.0 Public Dashboard of the National Pharmaceutical Pricing Authority (NPPA)
As on November 14, 2024 there are 32 registered PMRUs comprising 10 PMRUs under Category I, 9 PMRUs under category II and 13 PMRUs under Category III as per the Integrated Pharmaceutical Database Management System 2.0 Public Dashboard. Tamil Nadu under Category I and under Category III, Andaman & Nicobar Islands, Manipur, and Sikkim are yet to initiate PMRUs.
The need for the data on monitoring of prices along with other aspects was felt in the past, e.g., 54th Report of Standing Committee on Chemicals & Fertilizers (2018-19) on the subject ‘Pricing of Drugs with special reference to Drugs (Prices Control) Order, 2013’ that was presented to the Lok Sabha on February 13, 2019 had the comments of the Committee. The Committee had asked about the details of periodic survey conducted by the NPPA on prices of drugs sold in the country with particular reference to percentage of National List of Essential Medicines (NLEM) covered drugs/medicines in the market, it was stated that NPPA was collecting market price data from the agency named All India Organization of Chemists & Druggists & Advanced Working, Action and Correction System (AIOCD AWACS). Further, it was stated that NPPA had made agreement with the AIOCD AWACS who makes the collection of the data of the prices of the medicines, who collect the information from the majority of the distributors across the country. The information was provided on monthly basis. Based on analysis of the information submitted by the AIOCD-AWACS, NPPA monitored the prices of the medicines and issued notices for violation of the prices of medicines and therefore, objective of the survey used to be fulfilled.
The NPPA is entrusted with task of fixation of ceiling price of scheduled formulations (para 14 of the DPCO, 2013), revision of ceiling price of scheduled formulations (para 16), fixation of ceiling price of a drug under special circumstances (para 19), monitoring the prices of non-scheduled formulations (para 20), monitoring the availability of scheduled formulations (para 21), recovery of overcharged amount (para 23), among others. Each of these aspects is crucial in nature. For example, by June 30, 2022 the NPPA had fixed the ceiling prices of 890 scheduled formulations of medicines under the National List of Essential Medicines (NLEM) 2015. Regarding recovery of overcharged amount, during the financial year 2023-34 alone, Rs. 72.73 crore have been recovered from the defaulting companies wherever found selling formulations at prices higher than the permissible price.
Depth and breadth of pharmaceutical market can be gauged from the fact that during August 2019-July 2020 itself, there were 47,478 brands associated with 2,871 formulations in the pharmaceuticals market in India as per ‘Market Study on the Pharmaceutical Sector in India: Key Findings and Observations’, Competition Commission of India (CCI) dated November 18, 2021. Each therapeutic category has a huge number of formulations and brands, e.g., in Vitamins, Minerals and Nutrients category, there were 141 formulations and 5,236 brands thereby resulting into 37 brands per formulation. Same study cited 305 formulations in gastro-intestinal category with 5551 brands thereby having 18 brands per formulation. Currently, the number cited is more than 60,000 generic brands across 60 therapeutic categories in India.
The revised/modified scheme on setting Price Monitoring and Resource Units (PMRUs) at the State/UTs under the Central Sector Scheme of Consumer Awareness, Publicity and Price Monitoring was to be implemented in four years from the financial years 2020-21 through 2023-24. With most of these PMRUs in place, data will start pouring in to achieve objectives of price monitoring, along with comprehensive data on availability, accessibility and affordability of medicines.
Dr. Anil Kumar Angrish-Associate Professor (Finance and Accounting),Department of Pharmaceutical Management, NIPER S.A.S. Nagar (Mohali), Punjab
Disclaimer: Views are personal and do not represent the views of the Institute.





