The Indian Pharmaceutical Alliance (IPA) recently shared that the total number of the U.S. FDA inspections have declined globally to close to 940 in the year 2024 from about 1,849 annually in 2014. But for Indian pharmaceutical companies, the concern is that 18 per cent of global inspections were conducted in India in 2024, up from just 6 per cent of global inspections in 2014. It should also be seen in the background that Indian pharmaceutical companies now account for 40-45 per cent of the total generic drug volume in the US market.
The IPA represents 23 research-based pharmaceutical companies from India. IPA was formed as an association in August 1999 when six top pharmaceutical companies namely Cipla, Dr. Reddy’s Laboratories, Lupin, Piramal, Ranbaxy (part of Sun Pharma now), and Wockhardt joined as its founding members. Latest details show that members of IPA now have 85 per cent of the private sector investment in pharmaceutical R&D, 64 per cent share of the domestic market sales, 62 per cent share of price-controlled medicines, and 80 per cent share of India’s exports of drugs and pharmaceuticals.
Manufacturing facilities of pharmaceutical companies are certified by global regulatory agencies such as the USFDA, European Medicines Evaluation Agency (EMEA), UK Medicines and Healthcare Products Regulatory Agency (MHRA), Australia’s Therapeutic Goods Administration (TGA), Medicines Control Council (MCC) of South Africa, Federal Institute for Drugs and Medical Devices (BfArM) of Germany, Brazilian Health Regulatory Agency (ANVISA), the World Health Organization (WHO), South Korea’s Ministry of Food and Drug Safety, and Japan’s Pharmaceuticals and Medical Devices Agency to name the prominent ones. Hence, regulatory concerns of global regulatory agencies are of utmost importance as Indian pharmaceutical companies operate in almost all territories globally. The US market is the most important market for Indian pharmaceutical companies as the US forms about one-third of total export of drugs and pharmaceuticals from India.
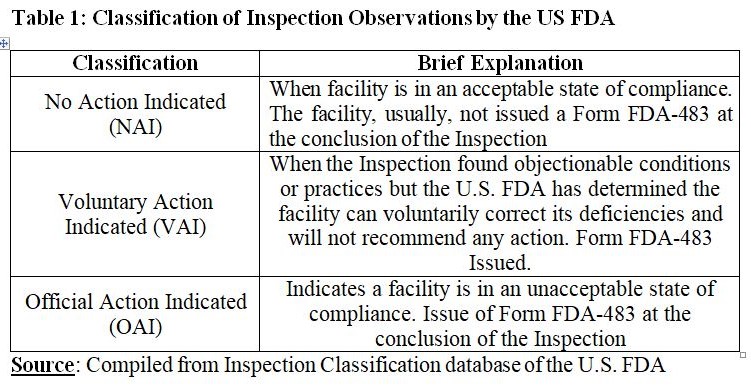
The U.S. FDA investigators list observed conditions or practices in Form FDA-483 Inspection Observations, Form FDA-4056 Produce Farm Inspection Observations, or notated in the Establishment Inspection Report. Further, inspected firms are encouraged to respond in writing in 15 days’ time, through submission of their corrective action plan with supporting documentation, and promptly implement corrective actions.
In 2023-24, Sun Pharma had pending USFDA compliance issues at three facilities comprising import alert at the Halol facility (Gujarat), the receipt of non-compliance letter for the Mohali facility (Punjab), both during FY23. The inspection classification status of Dadra facility (in Union Territory of Dadra and Nagar Haveli and Daman and Diu) was determined as Official Action Indicated (OAI) after inspection by the USFDA in December 2023. Till March 31, 2024, the company had 41 manufacturing facilities comprising 27 finished dosage manufacturing facilities and 14 API facilities, spanning six continents.
Divi’s Labs has two manufacturing facilities, and third one is in the set-up stage. The company is recognized as the largest API manufacturer in the world for 10 of the Generic APIs manufactured.
Cipla Limited had 46 manufacturing facilities in five countries by March 31, 2024. In FY24, Cipla underwent 52 regulatory inspections by major regulatory authorities. China facility of Cipla Limited that is expected to supply to the US cleared the USFDA Audit. The U.S. Food and Drug Administration conducted four inspections as manufacturing sites of the company in Fall River, Islip, Hauppauge (USA), and Qidong (China). The company reported that all these inspections were concluded without any observations except that it received five Form 483 observations for the inspection at Central Islip. These observations were subsequently classified as Voluntary Action Indicated (VAI). Regarding facilities in India, the company received a Warning Letter from the USFDA for cGMP inspection that was conducted at Indore site (Madhya Pradesh).
Torrent Pharma had 8 manufacturing facilities at various locations in India through which it caters 40+ countries across the world. In December 2023, Torrent Pharma’s Oncology Oral Solid manufacturing facility at Bileshwarpura underwent a USFDA Pre-approval Inspection (PAI) for capsule dosage forms. A Form FDA 483 with five procedural observations related to oncology product operations was issued by the USFDA. During FY24, the company’s Dahej facility got clearance from the USFDA. Two procedural observations were identified by the regulatory body during an inspection in May 2023 for which corrective actions were initiated by the company. Further, the company reported that the regulatory body issued an Establishment Inspection Report (EIR) in August 2023 through which the agency officially closed the inspection with a classification of Voluntary Action Indicated (VAI). During FY24, the company’s sales from the US business declined to US $128 Mn from US 138 Mn in FY23. Degrowth of 7 per cent was mainly attributed to lack of new products approval due to pending USFDA inspection of the company’s USFDA approved facilities.
By the end of FY24, Dr. Reddy’s Labs had 23 manufacturing facilities globally. The company reported that all of their facilities were fully compliant with USFDA regulations and were maintained to be ‘inspection ready’ for any regulator at all times. By March 31, 2024, the status for all other facilities was either NAI or VAI.
Lupin Limited has 15 global manufacturing sites, out of which, the company has 11 U.S. approved sites. The company addressed the warning letter from the U.S. FDA with regard to their facilities in Pithampur and Goa. The company is committed to target of zero warning letters/OAI issues by FY25. There were 9 U.S. FDA inspections (including GMP and Bioresearch Center) in FY23 and FY24. Number of Form 483 came down to 2 in FY24 from 7 in FY23. Number of Total Observations substantially came down from 55 in FY23 to just 3 in FY24. The U.S. FDA Inspection Warning Letters remained 1 in FY23 as well as FY24.
Zydus Lifesciences has 37 manufacturing facilities out of which 33 facilities are for manufacturing of finished dosage formulations and APIs. 16 out of 33 facilities have been inspected by the USFDA. In FY24, one facility in Ahmedabad SEZ received an Establishment Inspection Report with VAI status from the regulatory body against an inspection that was conducted in FY23. Facilities namely Biologics fill finish facility at Zydus Biotech Park, Animal health formulations manufacturing facility located in Ahmedabad SEZ and the newly constructed OSD facility II in Ahmedabad SEZ completed USFDA inspections without any observations.
By the end of FY24, Aurobindo Pharma had 29 commercial manufacturing facilities in India, Portugal, the US and Brazil. In FY24, six units received EIRs from US FDA. Inspection of Eugia-III unit of Eugia Pharma was concluded on February 2, 2024, that resulted in the identification of nine observations. There was a temporary pause of production line too. Corrective Action and Preventive Plan (CAPA) was submitted by the company.
By March 31, 2024, Alkem Labs had 19 manufacturing facilities. In FY24, three manufacturing facilities, i.e., Ankleshwar, Mandva, and Baddi, of Alkem Labs were inspected by the USFDA. Inspection of Ankleshwar facility was closed without any observations. Form 483 with 3 observations was received for Mandva facility and through EIR in March 2024, inspection was closed successfully. Form 483 with 10 observations was received for Baddi facility with no data integrity related observations to which the company submitted a detailed Corrective and Prevention Plan to get a closure of the inspection.
Glenmark Pharma has 4 manufacturing facilities approved by the US FDA out of 11 manufacturing facilities globally. In FY24, the company’s facilities underwent 32 regulatory inspections conducted by various regulatory bodies across different regions, up from 29 in FY23, 16 each in FY22 and FY21.
Top pharmaceutical companies have realized the benefits of being on the right side of regulation as reflected in the data pertaining to compliance with the U.S. FDA norms. India has the unique distinction of being a country that has the highest number of USFDA inspected plants outside the US, housing 25 per cent of US FDA-approved plants outside the U.S. With more product launches in the U.S. market, these pharmaceutical companies are likely to come under higher scrutiny. By addressing these concerns in a proactive manner through commitment to targets of zero warning letters or OAI issues can pave the way for smooth progress in the U.S. market. This will generate a positive signal for other regulatory bodies in different countries and help in building Bharat Brand.
Dr. Anil Kumar Angrish-Associate Professor (Finance and Accounting), Department of Pharmaceutical Management,NIPER S.A.S. Nagar (Mohali), Punjab
Disclaimer: Views are personal and do not represent the views of the Institute.





